March 4th, 2021
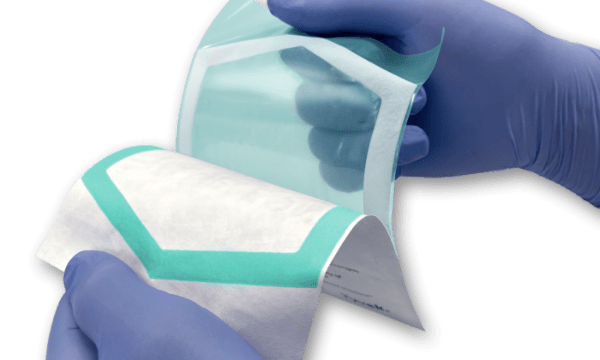
The next-generation Chameleon™ visual seal- assurance system was developed to support medical device manufacturers in complying with the new EU MDR guidelines that go into effect May of this year. 11.4 of the MDR’s General Safety and Performance Requirements (GSPR) requires that “the integrity of that packaging is clearly evident to the final user,” at the point-of-use. The advancement in Chameleon technology allows the end-user to readily evaluate the quality of the seal when used with uncoated Tyvek .