March 12th, 2021 | by Daphne Allen
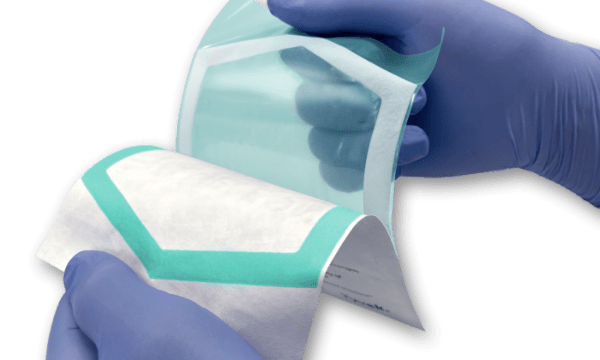
PAXXUS has launched a new sealant that can provide a vibrant, easy-to-see seal transfer on uncoated Tyvek. The EU Medical Device Regulation has heightened the importance of ensuring package usability. For instance, according to clause 11.4 of the MDR’s General Safety and Performance Requirements (GSPR), “it shall be ensured that the integrity of . . . packaging is clearly evident to the final user.” Medical device manufacturers marketing products in Europe are therefore expected to provide packaging that enables users to recognize a potential loss of integrity.