Technical Resource by on October 8, 2021
Topics covered:
-
- Europe’s recent advancements in device and packaging regulations
- Aligning MDR regulations with ISO standards
- Discovering a disconnect between regulations for medical devices and their packaging systems
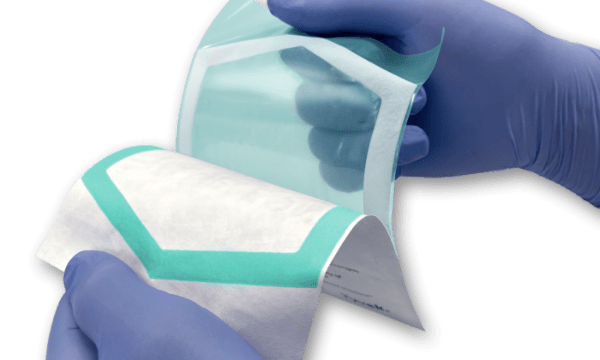
- Designing sterile packaging for successful end-use
Interested in learning more about this topic? Book a virtual lunch & learn session with the PAXXUS technical team!